| Section categories |
|
Related Subjects [38]
This category includes brief overview of all related subjects.
|
|
Defining BioInformatics [7]
In this section we tried to briefly explain what bioinformatics is ?
|
|
Unviersities [30]
This contains information about universities that are offering bioinformatics degree programs.
|
|
Resources [24]
Contains information about bioinformatics resources including databases, tools and techniques.
|
|
Algorithms [31]
This category includes some of the basic algorithms that are usually used by bioinformaticians.
|
|
| Statistics |
Total online: 1 Guests: 1 Users: 0 |
|
Home » 2011 » June » 20 » RER
|
How do proteins translocate into the lumen of the rough endoplasmic reticulum?
What is different about the protein that is destined for the rough endoplasmic reticulum?
The major difference is the fact that it has a hydrophobic signal sequence. This simplified cartoon shows that this is the first part of the protein produced. After the signal sequence is completed, protein synthesis is further inhibited. This is to allow the interaction of the signal sequence with a complex on the rough endoplasmic reticulum. In the above cartoon, note that the signal peptide is allowed to enter and essentially guide the protein into the lumen of the rough endoplasmic reticulum. Once the signal sequence is detected, protein synthesis resumes and the rest of the protein is inserted in the lumen. Note that a signal peptidase near the inner surface of the membrane works to cleave the signal sequence from the growing peptide.
The complex is actually more complicated than the above. The cartoon to the left shows a view of the signal sequence binding and interaction
Note that the signal sequence is recognized by a Recognition Particle, or SRP. This is then bound to a receptor. This complex guides the protein through a channel like region. It also consists of a docking site for the ribosome.
Another cartoon view of this process shows the signal receptor peptide (SRP) that associates with the large subunit of the ribosome that allows binding to the receptor on the rough endoplasmic reticulum.
After the protein is synthesized, the ribosome dissociates into large and small subunits and the SRP also looses its attachment to the receptor.
#Current studies of ribosomal interactions with ER:
Andrea Neuhof, M.M. Rolls, B. Jungnickel, K-U Kalies and T A Rapoport, Binding of Signal Recognition particle gives ribosome/nascent chain complexes a competitive advantage in endoplasmic reticulum membrane interaction. Molecular Biology of the Cell, 9: 103-115. 1997
- Proteins destined for RER sorting make a signal sequence.
- As signal sequence elongates, it is bound by the signal recognition particle (SRP54) (GTP dependent binding).
- SRP then binds to the SRP receptor (docking protein) on the ER membrane.
- At the same time, ribosomes bind to the RER translocation channel formed by the Sec61p complex.
- This Sec61p complex is a major component of a protein-conducting channel which also includes the "translocating chain-associating membrane protein” or TRAM. This conducts the protein into the RER sac.
Neuhof et al, 1997 have asked the following question: What stimulates the specificity of ribosomal docking?
- Question was asked because ribosomes dock to the Sec61p complex even without the signal sequence. Some clues:
- As the signal sequence begins to appear (short) it can be removed from the ER membrane with high salt concentrations.
- As the signal sequence elongates, Øribosome binding is stronger and ribosome complex becomes insensitive to proteases and high salt.
Neuhof's study hypothesized that the presence of the signal peptide was crucial for specific binding. Study Design:
üAdded nontranslating ribosomes to compete with translating ribosomes in an in vitro system.
- If Signal receptor protein (SRP) was absent, üthe nontranslating ribosomes bound to the Sec61p receptor on ER membranes.
- Also, the nontranslating ribosomes competed with the translating ribosomes.
Added SRP to bind to the signal peptide.
- The translating ribosomes bound tightly and were not displaced.
- Then, nontranslating ribosomes failed to compete for the Sec61p receptor sites.
Hence, SRP gives the translating ribosome a competitive edge once it starts translating the signal sequence.
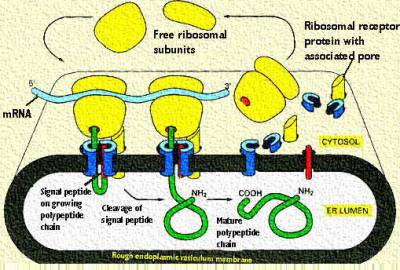
You can see a better surface view in this cartoon. The cartoon is from your text. It shows the Ribosome sitting on the receptor next to the pore. The signal peptide is noted in red. The remainder of the code is read as the ribosome moves along the mRNA. The fluidity of the membrane allows the ribosome to be docked at its receptor site and also move along the mRNA. Each amino acid is added to the growing chain and the polypeptide gets longer.
This cartoon is also from your text. It shows the system without the ribosome. Here you get a better view of the pore through which the protein projects into the lumen and the signal sequence.
|
|
|
Category: Related Subjects |
Views: 3624 |
Added by: Ansari
| Rating: 0.0/0 |
|
|
|




